gabriel amine synthesis
The Gabriel Synthesis of Primary Amines Prof. Gabriel a good friend of Emil Fischer often substituted for Fischer in his lectures.
We have seen how imide salts can be used for the synthesis of primary amines Gabriel.

. It is found that under basic conditions for the hydrolysis of N alkyl phthalimide the second step is the ratelimiting step. Structure of Amines Steps-. Hi allI have a question regarding the gabriel amine synthesis.
It has one hydrogenIf treated with an acid or a proton donor such as HCl or HI can the phtalimide can protonatedThis was an exam question scenario. Gabriel phthalimide synthesis is used for the preparation of aliphatic primary amines. A new and one-pot version of the Gabriel phthalimide amine synthesis utilizing carbonyl compounds as alkylating agents via their tosylhydrazone surrogates is disclosed.
This reaction is known as the Gabriel synthesis of primary amine. The alkylation step can take place either neat or in the presence of a solvent. Gabriel synthesis amines Where there are two carbonyl groups to stabilize the amide anion as in the l2-benzenedicarboximide phthalimide anion Section 18-IOC the acidity increases markedly and imides can be converted to their conjugate bases with concentrated aqueous hydroxide ion.
It used primary alkyl halides to prepare primary amines. Why can primary amines not be prepared by Gabriel synthesis. A color-coded approach to arrow pushing by Michael S.
I was jut wondering since all the gabriiel amine syntheses I have seen have involved the. Siegmund Gabriel 18511924 born in Berlin Germany studied under Hofmann at Berlin and Bunsen in Heidelberg. At the start you have a phthalimide molecule which is a secondary amine.
Secondary and tertiary amines are not formed by this method. A chemical process that converts primary alkyl halides to primary amines is known as the Gabriel synthesis. Previous article Next article.
Phenacylsulfonamides and triflamides with discussion of their generality and effectiveness. He taught at Berlin where he discovered the Gabriel synthesis of amines. It employs the use of a basic hydroxide and phthalimide.
Gabriel synthesis is used for primary amines. Traditionally potassium phthalimide is used in the reaction. Siegmund Gabriel a German chemist inspired the reactions name.
The basic hydroxide can be sodium hydroxide or potassium hydroxide. Gabriel synthesis The Gabriel synthesis named for the German chemist Siegmund Gabriel is a chemical reaction that transforms primary alkyl halides into. This video provides an overview of the Gabriel synthesis.
The method consists of alkylation of phthalimide anion with an appropriate alkylating reagent and subsequent removal of the phthaloyl group to generate primary amines Scheme 36. 226 PDF Tools Share Abstract Reaction of potassium phthalimide with halogenoalkanes and with a variety of other alkylating agents leads to the N-alkylphthalimide. So therefore to obtain pure and only 1-degree amine Gabriel phthalimide reaction is preferred.
Gabriel Phthalimide Synthesis reaction is a 3 step mechanism for the production of primary amide. The hydrolysis has been modified by using hydrazine. Amine that can not be prepared by Gabriel phthalimide synthesis is a benzylamineb anilinec methylamined iso-butyl aminee tertiary butylaminePW A.
The alkylation involves copper catalysed carbene insertion into the NH bond of phthalimide. Ueber eine Darstellung primärer Amine aus den entsprechenden Halogenverbindungen. Two new derivatives for this purpose are introduced.
It is an excerpt from the book Introductory Organic Reaction Mechanisms. The Gabriel synthesis is a classical but still useful procedure for the preparation of primary amines. The Gabriel synthesis is generalized as monoalkylation of an ammonia or primary amine derivative with subsequent removal of the derivatizing group s from nitrogen.
Henceforward the mild two-step process for the preparation of primary amines from their corresponding alkyl halides ie alkylation followed by hydrolysis became known as the Gabriel synthesis. The reaction is generally used for the synthesis of aliphatic primary amines.

Corey Fuchs Alkyne Synthesis En 2022 Quimica Organica Quimica Ciencia

L 9 Gabriel Phthalimide Synthesis Chemical Reaction With Important Points Neet Jee

Scheme 11 Organic Chemistry Science Chemistry Organic Chem

Pin On Reactions Of Carboxylic Acids And Their Derivatives Practice Problems

Pin On Reactions Of Carboxylic Acids And Their Derivatives Practice Problems

Gabriel Phthalimide Synthesis Mechanism Synthesis Of Primary Amine Scope Application Jam Net Gate
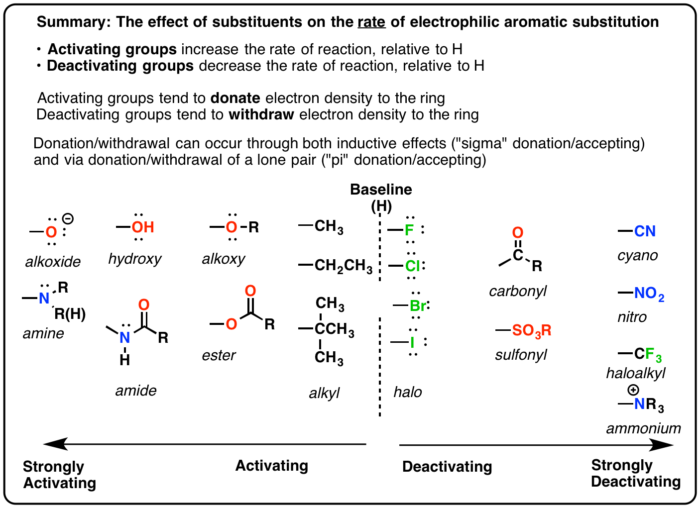
Activating And Deactivating Groups In Electrophilic Aromatic Substitution Aromatic Organic Chemistry Substitute

Fluorine And Amine Elimination Gives Hofmann Product Chemistry Lessons Organic Chemistry Chemistry

The Gabriel Synthesis Of Primary Amines Summary Of Methods For Hydrolysis Of The Imide Organic Chemistry Organic Chemistry Study Chemistry

Reductive Amination And How It Works Master Organic Chemistry Organic Chemistry Chemistry Writing Words

Gabriel Phthalimide Synthesis Mechanism Synthesis Of Primary Amine Scope Application Jam Net Gate

Naming Amine Choosing Parent Chain When There Is A Halogen Nomenclature Chemistry Chemistry Organic Chemistry Study

Complete Collection Organic Chemistry Organic Chemistry Notes Organic Chemistry Study

Naming A Primary Amine Systematic Names Nomenclature Chemistry Chemistry Organic Chemistry Reactions

Parikh Doering Oxidation Quimica Organica Quimica Matematicas Universitarias





Comments
Post a Comment